U.S. health authorities Monday announced plans to undertake a $36 million trial of an AIDS vaccine, the largest such trial to date
Published:
9 July 2002 y., Tuesday
About 16,000 HIV-negative volunteers will participate in the five-year study, to be co-sponsored by the U.S. National Institute of Allergy and Infectious Diseases and the Thai Ministry of Public Health.
Because it will take place in Thailand, the trial has been dubbed the "Thai Vaccine Trial."
Over six months, half of the participants will receive a series of three "primer" shots and two "booster" shots; the other half will receive placebo.
The "prime" part of the vaccine, called ALVAC-HIV, is made by Aventis Pasteur and the "boost," called AIDSVAX-B/E, is made by VaxGen.
Both medications have been studied independently and have generated strong safety records. Dr. Anthony Fauci, chairman of NIAID, expressed optimism.
Šaltinis:
cnn.com
Copying, publishing, announcing any information from the News.lt portal without written permission of News.lt editorial office is prohibited.
The most popular articles
 With the new influenza season underway, MEPs have criticised the EU's "disproportionate" response to the outbreak of the H1N1 ("swine flu") virus in 2009-2010.
more »
With the new influenza season underway, MEPs have criticised the EU's "disproportionate" response to the outbreak of the H1N1 ("swine flu") virus in 2009-2010.
more »
 Over half the EU adult population is now overweight or obese according to the “Health at a Glance: Europe 2010” report published by the European Commission and the OECD today.
more »
Over half the EU adult population is now overweight or obese according to the “Health at a Glance: Europe 2010” report published by the European Commission and the OECD today.
more »
 Over 130 people die in central Haiti due to a suspected outbreak of cholera.
more »
Over 130 people die in central Haiti due to a suspected outbreak of cholera.
more »
 The Influenza A (H1N1) pandemic returned to the Parliament last week but fortunately not in the literal sense.
more »
The Influenza A (H1N1) pandemic returned to the Parliament last week but fortunately not in the literal sense.
more »
 The Commission announced today its intention to restructure the process of progressive adoption of the list of permitted health claims on food products (also known as “Article 13 claims”).
more »
The Commission announced today its intention to restructure the process of progressive adoption of the list of permitted health claims on food products (also known as “Article 13 claims”).
more »
 Patients will be better informed on how to use medicines, and enabled to report their adverse effects directly to national authorities, thanks to updates of EU laws agreed with the Council and endorsed by Parliament on Wednesday.
more »
Patients will be better informed on how to use medicines, and enabled to report their adverse effects directly to national authorities, thanks to updates of EU laws agreed with the Council and endorsed by Parliament on Wednesday.
more »
 Doctors in Peru are facing outbreaks of two killer diseases, rabies and the plague, being spread by bats and rats.
more »
Doctors in Peru are facing outbreaks of two killer diseases, rabies and the plague, being spread by bats and rats.
more »
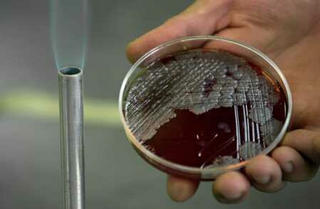 Scientists warn a new drug-resistant superbug could spread worldwide, fuelled in part by medical tourism.
more »
Scientists warn a new drug-resistant superbug could spread worldwide, fuelled in part by medical tourism.
more »
 Chinese officials say they are investigating reports that tainted milk powder has caused premature sexual development in baby girls.
more »
Chinese officials say they are investigating reports that tainted milk powder has caused premature sexual development in baby girls.
more »
 A woman in India says she has to sell her 6-month-old baby in order to pay her husband's medical expenses.
more »
A woman in India says she has to sell her 6-month-old baby in order to pay her husband's medical expenses.
more »